Main menu
- Bioethics Commission of University of Barcelona
- CBUB Technical Office
C/Baldiri i Reixac 2
2nd floor, Room 230
08028 Barcelona - Tel. ext 35 463
- cbub@ub.edu
- Office of the Vice Chancellor for Research
Rules to process reports
Rules to process reports for the Bioethics Committee from the Universitat de Barcelona
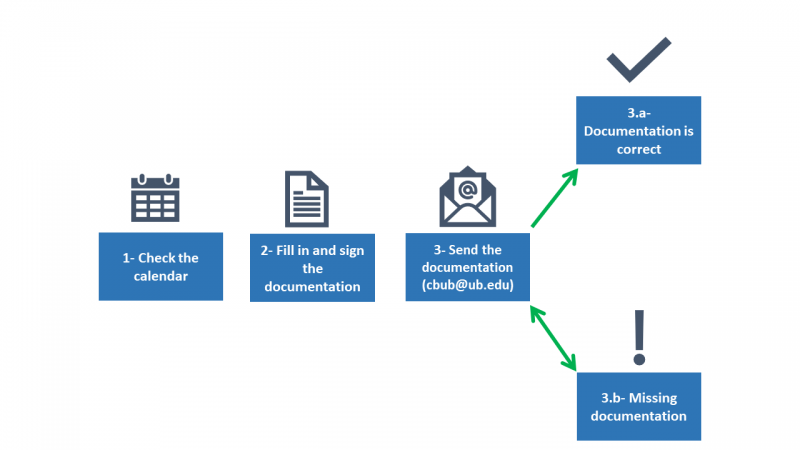
- Check on the calendar the deadline for submitting documentation for the next meeting.
- Fill in the appropriate form (Form 1, Form 2 or Form 3, depending on the nature of the research). Read carefully the documentation to be submitted as indicated on the forms and sign the required documentation. In the case of thesis/TFM/TFG, all documentation must be signed by both the director and/or tutor and the student. In the case of TFM and TFG, a letter signed by the tutor and the student stating that the project or work respects the ethical standards developed by the CBUB is required.
- Once the documentation is duly completed and signed, send it to CBUB by e-mail to cbub@ub.edu. Indicate in which language you would require the favorable report. Within a maximum period of one week, the reception of the correct and complete information would be confirmed.
3.a. If the documentation is correct, see point 4.
3.b. If documentation is missing, the Technical Secretary would request further information.
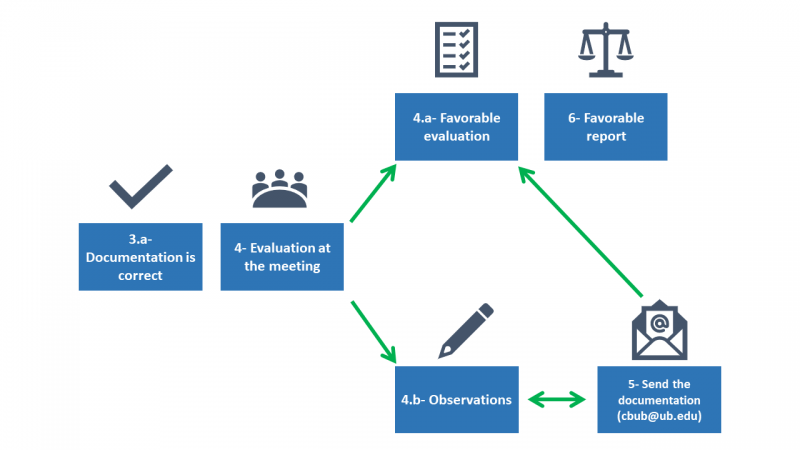
- Once the documentation is correct, its evaluation will take place at the next CBUB meeting (see calendar).
4.a. If the evaluation is favorable, the procedure to obtain the favorable report would be initiated with the approval of the CBUB president in the language indicated by the researcher.
4.b. If the project requires observations, it would be communicated by e-mail within a maximum period of two weeks from the date of the meeting.
- In the event that the project presents observations, the required documentation should be resubmitted in red or with change control activated to the e-mail cbub@ub.edu (check deadlines to present the amendments).
- Once the evaluation is favorable, the procedure indicated in point 4.a. will be followed.
You can download this document on PDF:
Diagram of the rules to process reports