An international team has developed a family of peptides that allow delta-9-tetrahydrocannabinol (THC), the main component of Cannabis sativa, to fight pain in mice without side effects. The study, published in the Journal of Medicinal Chemistry, counts with the participation of the coauthors Vicent Casadó and Estefanía Moreno, from the Molecular Neuropharmacology Research Group of the Faculty of Biology and the Institute of Biomedicine of the University of Barcelona (IBUB).
This research, led by Vicent Casadó and Estefanía Moreno (UB-IBUB), David Andreu and Rafael Maldonado (UPF), and Leonardo Pardo (UAB), was carried out together with researchers from Hospital del Mar Medical Research (IMIM) and University of Lisbon.
THC produces analgesia by binding to cannabinoid type 1 (CB1) receptors. However, these receptors interact with the serotonin receptor 5HT2A, and this interaction causes memory loss when THC is present.
«In the brain, these receptors associate and form complexes -heteromers- with serotonin receptor 5HT2A. However, the binding of THC to theses complexes causes memory deficits due to the interactions between the two receptors within the heteromer», explains Prof. Vicent Casadó, from the Department of Biochemistry and Molecular Biomedicine.
To prevent the activation of CB1 with cannabinoids to induce these memory problems while continuing to act as an analgesic, it is necessary to avoid or interfere with the formation of these receptor complexes by acting at the points of interaction with each other to form the heteromer.
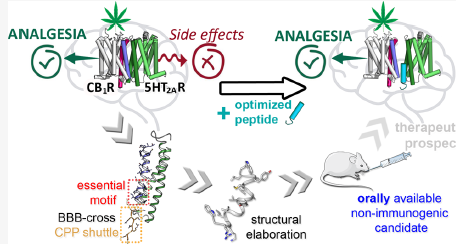
In a previous study carried out by the same research teams (PLOS Biology, 2015), they discovered the region of the two proteins involved in the formation of the heteromeric receptor complex. By adding a synthetic peptide having the sequence of this protein region, it is possible to interfere with the formation and / or function of the heteromer.
The aim of the present work was to optimize this peptide by reducing its size and increasing its plasma stability. Thus, the compound is able to cross the blood-brain barrier and prevent its immunogenic action in order to facilitate its oral administration in laboratory animals for later use in humans.
Specifically, the work of the Molecular Neuropharmacology Research Group team was focused on in vitro experiments to verify if the synthesized and successively optimized peptides maintain their interfering capacity between cannabinoid and serotonin receptors. As a result, the team has guided the process of optimization of the potential drug and has verified that, despite its reduction and chemical modification, the compound continues to interfere with the formation of the receptor heteromer and / or the allosteric communication between the receptors, responsible for the negative side effects on memory when CB1s are activated by cannabis derivatives.
Thus, this peptide would be an ideal candidate to reduce the cognitive side effects of pain treatment with cannabis derivatives. The results of the research have been the basis for an international patent application that is expected to be transferred to the pharmaceutical sector once the preclinical and clinical validation experiments required by drug regulations are completed.
Reference article
Gallo M et al. Orally Active Peptide Vector Allows Using Cannabis to Fight Pain While Avoiding Side Effects. Journal of Medicinal Chemistry. DOI: 10.1021/acs.jmedchem.1c00484.



