Capture and Separation of Gases by Adsorbent Materials
Capture and separation of gases and pollutants (CO2, N2, H2S, NOx, H2O, …) in sustainable industrial processes using absorbent solids (e.g., zeolites and metal-organic frameworks).
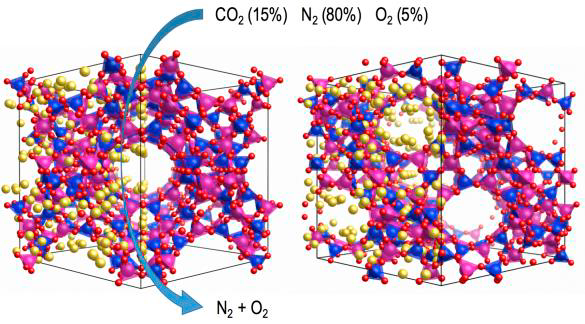
Description
Economic growth and industrial development have resulted in an increased burning of fossil fuels, leading to growing emissions of atmospheric CO2. These emissions may be reduced by a variety of measures, such as improving energy efficiency, and/or developing alternative energy sources, e.g. wind and solar power. However, the necessary transition into a sustainable energy mix, and the phasing out of fossil fuel combustion, is unlikely to occur at a sufficiently fast pace, unless additional, negative emission methods are considered. Reduction of energy-related CO2 emissions might be undertaken by means of Carbon Capture and Sequestration/Utilization (CCS/U) techniques. Separation technologies with proven adequacy for post-combustion processes are absorption, membrane use, and adsorption. Whereas the membrane technology is currently waiting its application to mass production, absorption is more mature, but it results in high-energy consumption during the absorbent regeneration step (i.e., about 30% of the output of the power plant).
Alternatively, CO2 can be captured through adsorption in the pores of solid materials like zeolites, activated carbons and metal-organic frameworks (MOFs). This promising adsorption technology is being investigated during the last years in our research group. Adsorption technology is based on the preferential affinity of CO2 to the adsorbent pores, compared to other flue gas components. After the adsorption step, molecules are desorbed from the solid by lowering the pressure (Pressure Swing Adsorption, PSA) or heating the solid material (Temperature Swing Adsorption, TSA) inside the column. The PSA process in which the desorption is performed below atmospheric pressure is called Vacuum Swing Adsorption (VSA). After this operation, the adsorbent is ready for a further cycle. All these methods have been used successfully for air fractionation, hydrogen production, carbon dioxide capture (CCS/U) and removal of volatile organic compounds (VOC). Among these methods, TSA is particularly promising, owing to difficulties with compressing or applying a vacuum to such large volumes of gas stream, as well as to the potential availability of low-grade heat in a power plant as a source of energy for regeneration.
Computational methods have been employed in a complementary fashion to experimental investigations. Grand-canonical Monte Carlo (GCMC) simulations allow the prediction of adsorption isotherms, adsorption selectivities and preferred adsorption sites at a very moderate computational expense, making an important contribution to the microscopic understanding of gas adsorption and separation in porous materials.
Collaborations
Dr. Fèlix Llovell (Institut Químic de Sarrià, IQC, Barcelona, Spain)
Fundings
MINECO (Project: CTQ2014-53987-R)

Generalitat de Catalunya (Project: 2014SGR1582)

