Our group is focused on the understanding of how the conformational dynamics of biomacromolecules affect the structure-activity relationships. I am interested in the development of new therapeutic strategies towards cardiometabolic diseases (CVDs) and other risk factors as type 2 diabetes and obesity.
Diabetes
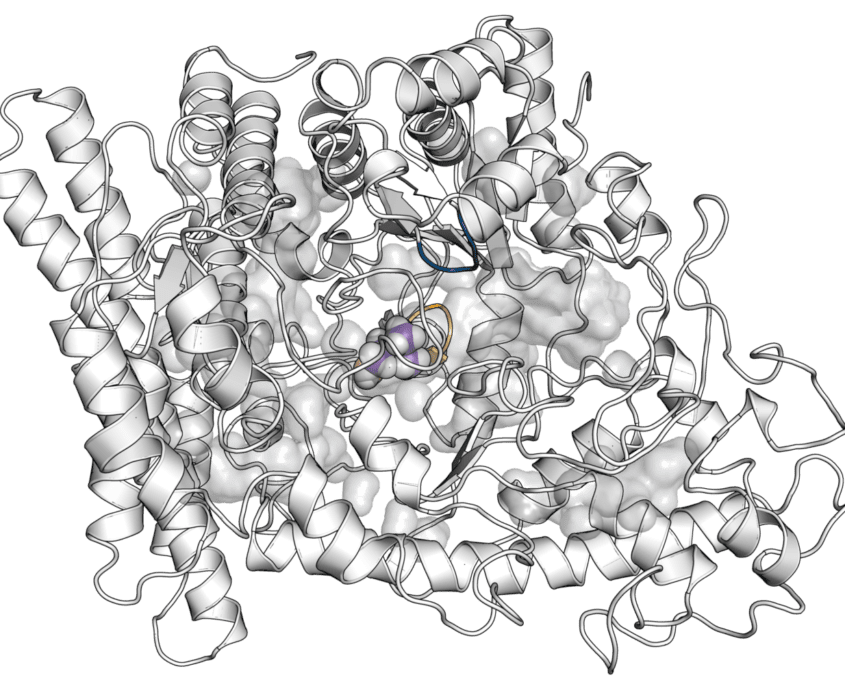
AMPK is a Ser/Thr protein kinase with a key role in cellular energy homeostasis.
It is a heterotrimeric complex consisting of a catalytic α-subunit and two regulatory (β and γ) components. These components are encoded by multiple genes, resulting in two α, two β and three γ isoforms that form up to 12 different complexes. These complexes have tissue-dependent expression, and exhibit notable differences in their specific activity, and in their sensitivity to allosteric activators.
The regulation of AMPK is finely controlled via several mechanisms: allosteric, indirect and direct. A direct activation of AMPK can be achieved by modulators that bind to the allosteric drug and metabolite (ADaM) site that is located at the interface between the α- and β-subunits, thus promoting an activation mechanism independent of Thr172 phosphorylation. As an example, binding of the thienopyridone activator A-769662 increases the AMPK activity >90-fold when Ser108 in the CBM domain of the β-subunit is phosphorylated. Several direct AMPK activators have been reported in the last few years. Strikingly, some of them exhibit marked isoform selectivity, while others show no isoform selectivity, and as such are labelled as pan-activators. The design of selective compounds that target specific AMPK subunit isoforms is an important research focal point, due to the specificity of their tissue distribution. Therefore, the search for new AMPK activators
with therapeutic potential for the treatment of metabolic diseases, having both vascular and metabolic beneficial effects, is needed.
More specifically, our principal objectives of this research line are to i) obtain a full a2b2g1 AMPK model, directly related to diabetes type-2, ii) design specific modulator for a2b2g1 AMPK model directly related to diabetes type-2, and iii) finally, our long-term project is related to develop a Machine Learning algorithm to disclose the allosteric network in AMPK, that can be subsequently extrapolated to other complex systems.

ADaM site: allosteric binding pocket in which direct modulators are bound
CBM domain: in b-subunit, where pSer108 is located.
P-loop at kinase domain: Important structural moiety for the design of allosteric molecular glues
choline binding site: Choline essential nutrient is located together with Cys loop shown in yellow
Glycyl radical domain: it contains the Glycyl Loop, suggested to fluctuate from open to close conformation
CutD approach: we have hypothesized that the activating-enzyme CutD, should approach to this region to start the radical reaction.
Cardiovascular Diseases (CDVs)
Recent studies have demonstrated that compositional and functional alterations in the gut microbiome have an impact on metabolic and cardiovascular diseases.
While these findings highlight the crucial role of the gut-heart axis, the results have disclosed the relevance of the microbiota in generating specific metabolites, like trimethylamine (TMA), with physio-pathological effects.
This research line aims to explore a novel therapeutic strategy for targeting CVDs, by investigating a promising enzyme implicated in the regulation of vascular function, the choline trimethylamine(TMA)-lyase, CutC, located at human gut microbiome. The final goal is to design a modulator of this enzyme that could help us to gain insight into the details of the gut-heart axis in CVDs, and more specifically to regulate the effect of gut microbiota dysbiosis in these patients, that is, design a modbiotic compound.
More specifically, our principal objectives of this research line are to i) understand the reaction mechanism at the active site of CutC by QM/MM studies; ii) obtain the 3D structure of CutD to explore the formation of the CutC:CutD complex, and iii) design an inhibitor to avoid the formation of TMA and TMAO metabolites. This last step could include the design of non-covalent and/or covalent inhibitor taking profit of the different paths that connect the active site with the outer space.
Obesity vs Alzheimer’s disease
The role of leptin resistance in obesity is well-known
However, the neuroprotective role that specific sequences of leptin protein have against two hallmark proteins related to Alzheimer’s disease remains to be elucidated.
Alzheimers Disease (AD) is the most common form of dementia. Currently, there is no cure for AD and very few therapies are available to alleviate symptoms. Peptide-based therapeutics offer many advantages such as their high selectivity, potency and low toxicity. Our collaborators have recently shown that leptin peptides are potential therapeutics in the fight against AD. This provided the first compelling evidence for the neuroprotective effects of small, leptin-derived peptides which represents a possibility for breakthrough. However, per se, leptin peptides cannot be administered in tablet form as they are rapidly broken down by the digestive system. To translate these findings to a therapeutic advance requires refinements to stabilize these molecules permitting end-user friendly administration whilst maintaining biological activity.In Pep-AD-designer project, we propose to use an iterative approach, combining synthetic and computational chemistry with in vitro bioassays, to design novel bioactive leptin-based peptides with improved pharmacokinetics. Through this interdisciplinary approach, Pep-AD-designer project will address the short-comings of leptin-based peptide therapeutics to bring this technology closer to robust in vivo tests and clinical trials, transforming biologic drug development. Finally, we envisage the development of a patentable, effective method to stabilise biological therapeutics.
Our current objective is to understand the mode of action of these specific peptides against the self-aggregation of Ab-amyloid and tau proteins. This project is developed together with Prof. C. Pubill, PIs of the Proyecto Generacion de Conocimiento 2023 (PID2022-142623OA-I00). We oversee the computational studies that will be used to improve the design of these peptides with enhanced pharmacokinetics properties.
QM Skills…
We use Quantum Mechanics (QM) computations for the full characterization of small organic molecules, like minima optimization, determination of transition states or the calculation of different physicochemical properties in gas and implicit solvents.
Likewise, we have used quantum semiempirical calculations, DFT and post-HF methods for the understanding of the interplay between different noncovalent interactions or the determination of the reaction mechanisms.
Additionally, due to the study of different metalloproteins and IDPs, we have developed a key understanding of re-parameterization of complex systems, helping us to modify specifically some force field features.
MM Skills…
We use Molecular Mechanics (MM) simulations, mainly Molecular Dynamics simulations to characterize the protein dynamics in absence and in presence of small organic molecules or peptides. Our main effort is on the understanding of the structural dynamics of complex heteromers systems to understand the effect over its activity. With this information, our aim is to design a new bioactive compound determining the hotspots of the active site or design a new therapeutic strategies based on allosteric pockets determining the network communication.
Additionally, we have focused our attention on the folding and aggregation process of small peptides, and the binding of small ligands to PPIs where the use of enhanced sampling techniques, like FEPs, Metadynamics or aMD have been widely used by the group.
¿Rogamus, igitur, vos et monemus attencius?
Et sub ea qua possumus vobis districcione mandamus, quatenus domui Populetensi vel hominibus suis de Avimbodi aut quibuslibet eiusdem bonis malum aliquod vel gravamen non attempetis inferre aut domum illam vel sua aliqua aliquatenus molestare, si de gracia nostra spem aut fiduciam bonam de cetero habere vobis confiditis aut speratis.
¿In dampno enim illius domus et gravamine gravaremur et moveremur?
¿Qué es ?
Lorem
¿Quia vero litere iste non sunt sigillate sigillo nostro?
Rogamus, igitur, vos et monemus attencius et sub ea qua possumus vobis districcione mandamus, quatenus domui Populetensi vel hominibus suis de Avimbodi aut quibuslibet eiusdem bonis malum aliquod vel gravamen non attempetis inferre aut domum illam vel sua aliqua aliquatenus molestare, si de gracia nostra spem aut fiduciam bonam de cetero habere vobis confiditis aut speratis.
And new ones with every update
who trust Enfold with their site
You got any issues? Get in touch!