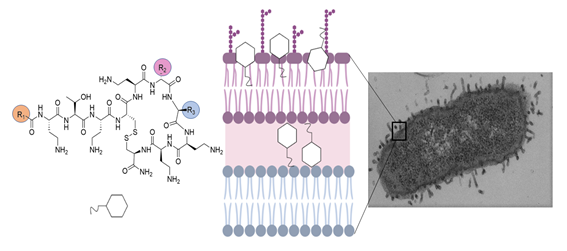
Antibiotic resistance is a major public health challenge, and infections caused by Gram-negative multi-drug resistant bacteria are considered an unmet medical need. The threat of running out of active molecules is accelerated by the extensive use of antibiotics in the context of the COVID-19 pandemic, and new antibiotics are urgently needed. The group of Peptides and Proteins: Physicochemical Studies in collaboration with researchers from the Faculty of Chemistry, UB, design and synthesize compounds inspired in natural polymyxins with a flexible scaffold that allows multiple modifications to improve activity and reduce toxicity. In this work, we focus on modifications in the hydrophobic domains, obtaining analogues with different spectrum of activity, MICs in the low µM range and low hemolytic activity. Redistribution of the hydrophobicity within the polymyxin molecule seems a plausible approach for the design and development of safer and more selective antibiotics.
Unveiling the Membrane and Cell Wall Action of Antimicrobial Cyclic Lipopeptides: Modulation of the Spectrum of Activity. R. Segovia, J. Solé, AM. Marqués, Y. Cajal and F. Rabanal. Pharmaceutics 2021, 13(12)