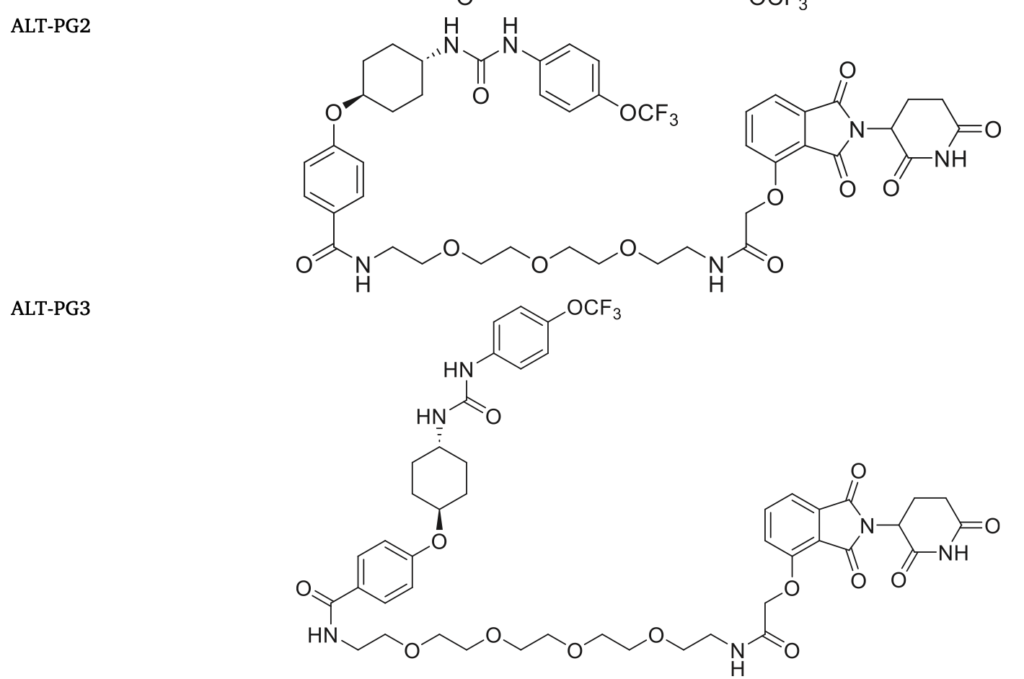
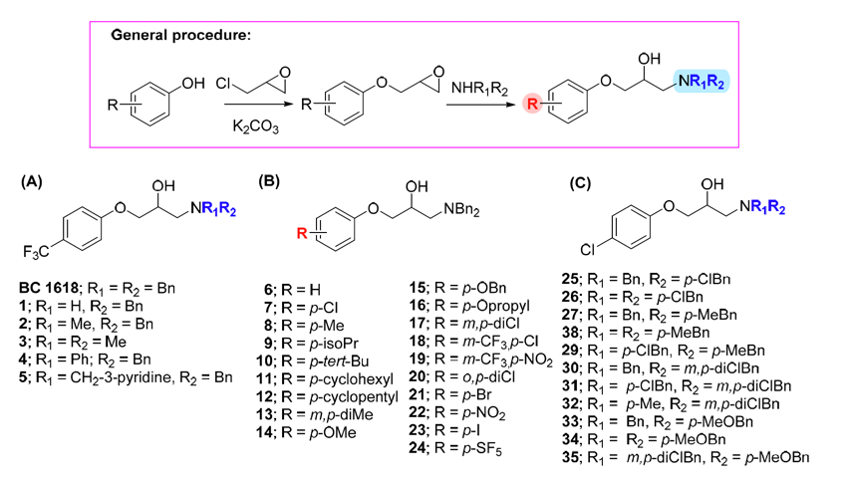
Bioorg. Med. Chem. 27:115078, 2019.
Abstract.
Soluble epoxide hydrolase (sEH) inhibitors are potential drugs for several diseases. Adamantyl ureas are excellent sEH inhibitors but have limited metabolic stability. Herein, we report the effect of replacing the adamantane group by alternative polycyclic hydrocarbons on sEH inhibition, solubility, permeability and metabolic stability. Compounds bearing smaller or larger polycyclic hydrocarbons than adamantane yielded all good inhibition potency of the human sEH (0.4 ≤ IC50 ≤ 21.7 nM), indicating that sEH is able to accommodate inhibitors of very different size. Human liver microsomal stability of diamantane containing inhibitors is lower than that of their corresponding adamantane counterparts.