Currently, a great optimism exists concerning the therapeutic opportunities of drugs targeting both ionotropic and metabotropic neurotransmitter receptors. Indeed, recent research has focused on elucidating the role of posttranslational modifications and protein-protein interactions (i.e. oligomerization) in the cell surface targeting and function of ionotropic and/or G protein-coupled receptors. Hence, the development of either single or combined (i.e. multimodal) therapies able to finely modulate neurotransmitter receptors involved in such modifications/interactions would overcome classical therapeutic approaches, in which these complex connections were not initially considered. A paradigmatic example of this innovative hypothesis consists of the study of dopamine/adenosine receptor oligomerization, which is expected to provide a new generation of drugs with immediate application in some neurological diseases in general and in schizophrenia and Parkinson’s disease (PD) in particular.
In theory, multimodal therapies targeting oligomeric receptors may offer different therapeutic outputs to those expected for singlehanded drugs. In this sense, the increased knowledge about receptor oligomerization has propelled the development of drugs for PD treatment. Indeed, adenosine A2A receptor (A2AR) antagonists have been shown to possess antiparkinsonian properties in human PD patients. Despite the demonstration of adenosine receptor-containing oligomers in striatal neurons adds further complexity to the understanding of striatopallidal neurochemistry, it may result very valuable given the new potential therapeutic opportunities. On the other hand, mutations and posttranslational modifications affecting N-methyl-D-aspartate glutamate receptors (NMDARs) cell surface expression and function have been associated to several neuronal dysfunctions and synaptopathies such as Alzheimer's disease (AD), Down syndrome (DS) and Intellectual disability (ID), within others. Thus, specific pharmacological interventions modulating the surface density and/or the activity of NMDAR might improve the pharmacotherapy of these conditions.
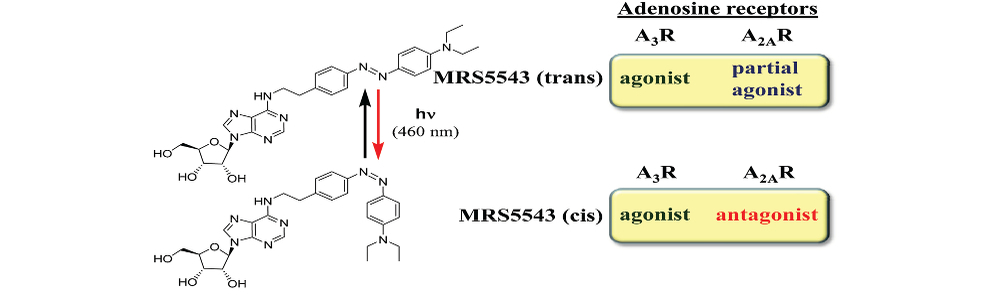
In pharmacology, a major impediment to identify the intrinsic efficacy of cell surface receptor drugs could be the lack of unambiguous biological assays to monitor receptor activity. For GPCRs and ion channels, this should not constitute a major concern, since several signalling pathways and biophysical approaches allow monitoring receptor activity upon drug challenge. Indeed, GPCR screening technologies include receptor binding, G-protein dependent and G-protein independent assays. On the other hand, for membrane receptors with ambiguous signalling pathways (i.e. sigma-1 receptor) and for GPCR oligomers, the screening of drugs by means of functional assays is not easy, and mostly relies on indirect biological determinations. Therefore, ascertaining the intrinsic activity of drugs targeting these kinds of signalling complexes is still a great challenge for modern pharmacology. Accordingly, to answer such pharmacological questions we work on engineering membrane receptors biosensors to monitor early events (i.e. receptor conformational changes) associated to the drug-receptor interaction processes. Finally, another important issue revolving receptors biology consists of the spatiotemporal manipulation of these receptors, a subject that may be somehow answered with optopharmacological methods.