Miñarro-Lleonar, Marina; Bertran-Mostazo, Andrea; Duro, Jorge; Barril, Xavier; Juárez-Jiménez, Jordi Lenalidomide Stabilizes Protein–Protein Complexes by Turning Labile Intermolecular H-Bonds into Robust Interactions (Journal Article) In: Journal of Medicinal Chemistry, vol. 66, no. 9, pp. 6037–6046, 2023, ISSN: 1520-4804. @article{Mi_arro_Lleonar_2023,
title = {Lenalidomide Stabilizes Protein–Protein Complexes by Turning Labile Intermolecular H-Bonds into Robust Interactions},
author = {Marina Miñarro-Lleonar and Andrea Bertran-Mostazo and Jorge Duro and Xavier Barril and Jordi Juárez-Jiménez},
url = {http://dx.doi.org/10.1021/acs.jmedchem.2c01692},
doi = {10.1021/acs.jmedchem.2c01692},
issn = {1520-4804},
year = {2023},
date = {2023-04-01},
urldate = {2023-04-01},
journal = {Journal of Medicinal Chemistry},
volume = {66},
number = {9},
pages = {6037–6046},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Scott, Jamie I.; Mendive-Tapia, Lorena; Gordon, Doireann; Barth, Nicole D.; Thompson, Emily J.; Cheng, Zhiming; Taggart, David; Kitamura, Takanori; Bravo-Blas, Alberto; Roberts, Edward W.; Juarez-Jimenez, Jordi; Michel, Julien; Piet, Berber; Vries, I. Jolanda; Verdoes, Martijn; Dawson, John; Carragher, Neil O.; Connor, Richard A. O’; Akram, Ahsan R.; Frame, Margaret; Serrels, Alan; Vendrell, Marc A fluorogenic probe for granzyme B enables in-biopsy evaluation and screening of response to anticancer immunotherapies (Journal Article) In: Nature Communications, vol. 13, no. 1, 2022, ISSN: 2041-1723. @article{Scott_2022,
title = {A fluorogenic probe for granzyme B enables in-biopsy evaluation and screening of response to anticancer immunotherapies},
author = {Jamie I. Scott and Lorena Mendive-Tapia and Doireann Gordon and Nicole D. Barth and Emily J. Thompson and Zhiming Cheng and David Taggart and Takanori Kitamura and Alberto Bravo-Blas and Edward W. Roberts and Jordi Juarez-Jimenez and Julien Michel and Berber Piet and I. Jolanda Vries and Martijn Verdoes and John Dawson and Neil O. Carragher and Richard A. O’ Connor and Ahsan R. Akram and Margaret Frame and Alan Serrels and Marc Vendrell},
url = {http://dx.doi.org/10.1038/s41467-022-29691-w},
doi = {10.1038/s41467-022-29691-w},
issn = {2041-1723},
year = {2022},
date = {2022-05-01},
journal = {Nature Communications},
volume = {13},
number = {1},
publisher = {Springer Science and Business Media LLC},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Codony, Sandra; Pont, Caterina; Griñán-Ferré, Christian; Pede-Mattatelli, Ania Di; Calvó-Tusell, Carla; Feixas, Ferran; Osuna, Sílvia; Jarné-Ferrer, Júlia; Naldi, Marina; Bartolini, Manuela; Loza, María Isabel; Brea, José; Pérez, Belén; Bartra, Clara; Sanfeliu, Coral; Juárez-Jiménez, Jordi; Morisseau, Christophe; Hammock, Bruce D.; Pallàs, Mercè; Vázquez, Santiago; Muñoz-Torrero, Diego Discovery and In Vivo Proof of Concept of a Highly Potent Dual Inhibitor of Soluble Epoxide Hydrolase and Acetylcholinesterase for the Treatment of Alzheimer’s Disease (Journal Article) In: Journal of Medicinal Chemistry, vol. 65, no. 6, pp. 4909–4925, 2022, ISSN: 1520-4804. @article{Codony_2022,
title = {Discovery and In Vivo Proof of Concept of a Highly Potent Dual Inhibitor of Soluble Epoxide Hydrolase and Acetylcholinesterase for the Treatment of Alzheimer’s Disease},
author = {Sandra Codony and Caterina Pont and Christian Griñán-Ferré and Ania Di Pede-Mattatelli and Carla Calvó-Tusell and Ferran Feixas and Sílvia Osuna and Júlia Jarné-Ferrer and Marina Naldi and Manuela Bartolini and María Isabel Loza and José Brea and Belén Pérez and Clara Bartra and Coral Sanfeliu and Jordi Juárez-Jiménez and Christophe Morisseau and Bruce D. Hammock and Mercè Pallàs and Santiago Vázquez and Diego Muñoz-Torrero},
url = {http://dx.doi.org/10.1021/acs.jmedchem.1c02150},
doi = {10.1021/acs.jmedchem.1c02150},
issn = {1520-4804},
year = {2022},
date = {2022-03-01},
journal = {Journal of Medicinal Chemistry},
volume = {65},
number = {6},
pages = {4909–4925},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Kowalczyk, Joanna; Grapsi, Ettore; Espargaró, Alba; Caballero, Ana B.; Juárez-Jiménez, Jordi; Busquets, Maria A.; Gamez, Patrick; Sabate, Raimon; Estelrich, Joan Dual Effect of Prussian Blue Nanoparticles on Aβ40 Aggregation: β-Sheet Fibril Reduction and Copper Dyshomeostasis Regulation (Journal Article) In: Biomacromolecules, vol. 22, no. 2, pp. 430–440, 2021, ISSN: 1526-4602. @article{Kowalczyk_2021,
title = {Dual Effect of Prussian Blue Nanoparticles on Aβ40 Aggregation: β-Sheet Fibril Reduction and Copper Dyshomeostasis Regulation},
author = {Joanna Kowalczyk and Ettore Grapsi and Alba Espargaró and Ana B. Caballero and Jordi Juárez-Jiménez and Maria A. Busquets and Patrick Gamez and Raimon Sabate and Joan Estelrich},
url = {http://dx.doi.org/10.1021/acs.biomac.0c01290},
doi = {10.1021/acs.biomac.0c01290},
issn = {1526-4602},
year = {2021},
date = {2021-01-01},
journal = {Biomacromolecules},
volume = {22},
number = {2},
pages = {430–440},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
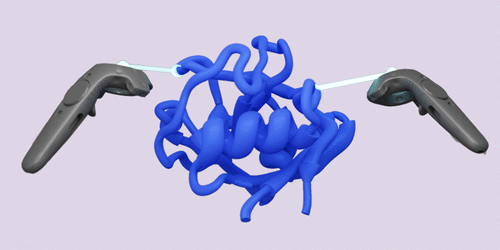
Juárez-Jiménez, Jordi; Tew, Philip; O′Connor, Michael; Llabrés, Salomé; Sage, Rebecca; Glowacki, David; Michel, Julien Combining Virtual Reality Visualization with Ensemble Molecular Dynamics to Study Complex Protein Conformational Changes (Journal Article) In: Journal of Chemical Information and Modeling, vol. 60, no. 12, pp. 6344–6354, 2020, ISSN: 1549-960X. @article{Ju_rez_Jim_nez_2020,
title = {Combining Virtual Reality Visualization with Ensemble Molecular Dynamics to Study Complex Protein Conformational Changes},
author = {Jordi Juárez-Jiménez and Philip Tew and Michael O′Connor and Salomé Llabrés and Rebecca Sage and David Glowacki and Julien Michel},
url = {http://dx.doi.org/10.1021/acs.jcim.0c00221},
doi = {10.1021/acs.jcim.0c00221},
issn = {1549-960X},
year = {2020},
date = {2020-11-01},
urldate = {2020-11-01},
journal = {Journal of Chemical Information and Modeling},
volume = {60},
number = {12},
pages = {6344–6354},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Kuzmanic, Antonija; Bowman, Gregory R.; Juarez-Jimenez, Jordi; Michel, Julien; Gervasio, Francesco L. Investigating Cryptic Binding Sites by Molecular Dynamics Simulations (Journal Article) In: Accounts of Chemical Research, vol. 53, no. 3, pp. 654–661, 2020, ISSN: 1520-4898. @article{Kuzmanic_2020,
title = {Investigating Cryptic Binding Sites by Molecular Dynamics Simulations},
author = {Antonija Kuzmanic and Gregory R. Bowman and Jordi Juarez-Jimenez and Julien Michel and Francesco L. Gervasio},
url = {http://dx.doi.org/10.1021/acs.accounts.9b00613},
doi = {10.1021/acs.accounts.9b00613},
issn = {1520-4898},
year = {2020},
date = {2020-03-01},
journal = {Accounts of Chemical Research},
volume = {53},
number = {3},
pages = {654–661},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
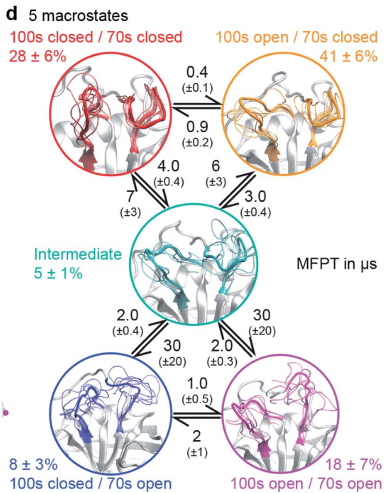
Juárez-Jiménez, Jordi; Gupta, Arun A.; Karunanithy, Gogulan; Mey, Antonia S. J. S.; Georgiou, Charis; Ioannidis, Harris; Simone, Alessio De; Barlow, Paul N.; Hulme, Alison N.; Walkinshaw, Malcolm D.; Baldwin, Andrew J.; Michel, Julien Dynamic design: manipulation of millisecond timescale motions on the energy landscape of cyclophilin A (Journal Article) In: Chemical Science, vol. 11, no. 10, pp. 2670–2680, 2020, ISSN: 2041-6539. @article{Ju_rez_Jim_nez_2020b,
title = {Dynamic design: manipulation of millisecond timescale motions on the energy landscape of cyclophilin A},
author = {Jordi Juárez-Jiménez and Arun A. Gupta and Gogulan Karunanithy and Antonia S. J. S. Mey and Charis Georgiou and Harris Ioannidis and Alessio De Simone and Paul N. Barlow and Alison N. Hulme and Malcolm D. Walkinshaw and Andrew J. Baldwin and Julien Michel},
url = {http://dx.doi.org/10.1039/C9SC04696H},
doi = {10.1039/c9sc04696h},
issn = {2041-6539},
year = {2020},
date = {2020-01-01},
urldate = {2020-01-01},
journal = {Chemical Science},
volume = {11},
number = {10},
pages = {2670–2680},
publisher = {Royal Society of Chemistry (RSC)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Simone, Alessio De; Georgiou, Charis; Ioannidis, Harris; Gupta, Arun A.; Juárez-Jiménez, Jordi; Doughty-Shenton, Dahlia; Blackburn, Elizabeth A.; Wear, Martin A.; Richards, Jonathan P.; Barlow, Paul N.; Carragher, Neil; Walkinshaw, Malcolm D.; Hulme, Alison N.; Michel, Julien A computationally designed binding mode flip leads to a novel class of potent tri-vector cyclophilin inhibitors (Journal Article) In: Chemical Science, vol. 10, no. 2, pp. 542–547, 2019, ISSN: 2041-6539. @article{De_Simone_2019,
title = {A computationally designed binding mode flip leads to a novel class of potent tri-vector cyclophilin inhibitors},
author = {Alessio De Simone and Charis Georgiou and Harris Ioannidis and Arun A. Gupta and Jordi Juárez-Jiménez and Dahlia Doughty-Shenton and Elizabeth A. Blackburn and Martin A. Wear and Jonathan P. Richards and Paul N. Barlow and Neil Carragher and Malcolm D. Walkinshaw and Alison N. Hulme and Julien Michel},
url = {http://dx.doi.org/10.1039/C8SC03831G},
doi = {10.1039/c8sc03831g},
issn = {2041-6539},
year = {2019},
date = {2019-01-01},
journal = {Chemical Science},
volume = {10},
number = {2},
pages = {542–547},
publisher = {Royal Society of Chemistry (RSC)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Alencar, Nelson; Sola, Irene; Linares, María; Juárez-Jiménez, Jordi; Pont, Caterina; Viayna, Antonio; Vílchez, David; Sampedro, Cristina; Abad, Paloma; Pérez-Benavente, Susana; Lameira, Jerónimo; Bautista, José M.; Muñoz-Torrero, Diego; Luque, F. Javier First homology model of Plasmodium falciparum glucose-6-phosphate dehydrogenase: Discovery of selective substrate analog-based inhibitors as novel antimalarial agents (Journal Article) In: European Journal of Medicinal Chemistry, vol. 146, pp. 108–122, 2018, ISSN: 0223-5234. @article{Alencar_2018,
title = {First homology model of Plasmodium falciparum glucose-6-phosphate dehydrogenase: Discovery of selective substrate analog-based inhibitors as novel antimalarial agents},
author = {Nelson Alencar and Irene Sola and María Linares and Jordi Juárez-Jiménez and Caterina Pont and Antonio Viayna and David Vílchez and Cristina Sampedro and Paloma Abad and Susana Pérez-Benavente and Jerónimo Lameira and José M. Bautista and Diego Muñoz-Torrero and F. Javier Luque},
url = {http://dx.doi.org/10.1016/j.ejmech.2018.01.044},
doi = {10.1016/j.ejmech.2018.01.044},
issn = {0223-5234},
year = {2018},
date = {2018-02-01},
journal = {European Journal of Medicinal Chemistry},
volume = {146},
pages = {108–122},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Mey, Antonia S. J. S.; Jiménez, Jordi Juárez; Michel, Julien Impact of domain knowledge on blinded predictions of binding energies by alchemical free energy calculations (Journal Article) In: Journal of Computer-Aided Molecular Design, vol. 32, no. 1, pp. 199–210, 2017, ISSN: 1573-4951. @article{Mey_2017,
title = {Impact of domain knowledge on blinded predictions of binding energies by alchemical free energy calculations},
author = {Antonia S. J. S. Mey and Jordi Juárez Jiménez and Julien Michel},
url = {http://dx.doi.org/10.1007/s10822-017-0083-9},
doi = {10.1007/s10822-017-0083-9},
issn = {1573-4951},
year = {2017},
date = {2017-11-01},
journal = {Journal of Computer-Aided Molecular Design},
volume = {32},
number = {1},
pages = {199–210},
publisher = {Springer Science and Business Media LLC},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Llona-Minguez, Sabin; Fayezi, Shabnam; Alihemmati, Alireza; Juárez-Jiménez, Jordi; Piedrafita, F. Javier; Helleday, Thomas Tetrahydrobenzothiophene carboxamides: Beyond the kinase domain and into the fatty acid realm (Journal Article) In: Bioorganic & Medicinal Chemistry Letters, vol. 27, no. 18, pp. 4462–4466, 2017, ISSN: 0960-894X. @article{Llona_Minguez_2017,
title = {Tetrahydrobenzothiophene carboxamides: Beyond the kinase domain and into the fatty acid realm},
author = {Sabin Llona-Minguez and Shabnam Fayezi and Alireza Alihemmati and Jordi Juárez-Jiménez and F. Javier Piedrafita and Thomas Helleday},
url = {http://dx.doi.org/10.1016/j.bmcl.2017.08.006},
doi = {10.1016/j.bmcl.2017.08.006},
issn = {0960-894X},
year = {2017},
date = {2017-09-01},
journal = {Bioorganic & Medicinal Chemistry Letters},
volume = {27},
number = {18},
pages = {4462–4466},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Pietro, Ornella Di; Juárez-Jiménez, Jordi; Muñoz-Torrero, Diego; Laughton, Charles A.; Luque, F. Javier Unveiling a novel transient druggable pocket in BACE-1 through molecular simulations: Conformational analysis and binding mode of multisite inhibitors (Journal Article) In: PLOS ONE, vol. 12, no. 5, pp. e0177683, 2017, ISSN: 1932-6203. @article{Di_Pietro_2017,
title = {Unveiling a novel transient druggable pocket in BACE-1 through molecular simulations: Conformational analysis and binding mode of multisite inhibitors},
author = {Ornella Di Pietro and Jordi Juárez-Jiménez and Diego Muñoz-Torrero and Charles A. Laughton and F. Javier Luque},
editor = {Chandra Verma},
url = {http://dx.doi.org/10.1371/journal.pone.0177683},
doi = {10.1371/journal.pone.0177683},
issn = {1932-6203},
year = {2017},
date = {2017-05-01},
journal = {PLOS ONE},
volume = {12},
number = {5},
pages = {e0177683},
publisher = {Public Library of Science (PLoS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Delcanale, P.; Rodríguez-Amigo, B.; Juárez-Jiménez, J.; Luque, F. J.; Abbruzzetti, S.; Agut, M.; Nonell, S.; Viappiani, C. Tuning the local solvent composition at a drug carrier surface: the effect of dimethyl sulfoxide/water mixture on the photofunctional properties of hypericin–β-lactoglobulin complexes (Journal Article) In: Journal of Materials Chemistry B, vol. 5, no. 8, pp. 1633–1641, 2017, ISSN: 2050-7518. @article{Delcanale_2017,
title = {Tuning the local solvent composition at a drug carrier surface: the effect of dimethyl sulfoxide/water mixture on the photofunctional properties of hypericin–β-lactoglobulin complexes},
author = {P. Delcanale and B. Rodríguez-Amigo and J. Juárez-Jiménez and F. J. Luque and S. Abbruzzetti and M. Agut and S. Nonell and C. Viappiani},
url = {http://dx.doi.org/10.1039/C7TB00081B},
doi = {10.1039/c7tb00081b},
issn = {2050-7518},
year = {2017},
date = {2017-01-01},
journal = {Journal of Materials Chemistry B},
volume = {5},
number = {8},
pages = {1633–1641},
publisher = {Royal Society of Chemistry (RSC)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
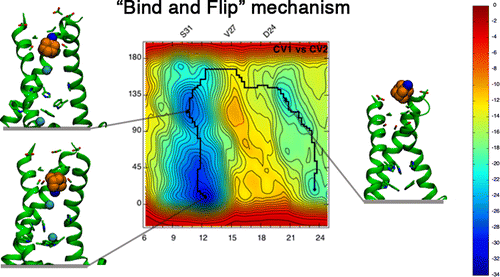
Llabrés, Salomé; Juárez-Jiménez, Jordi; Masetti, Matteo; Leiva, Rosana; Vázquez, Santiago; Gazzarrini, Sabrina; Moroni, Anna; Cavalli, Andrea; Luque, F. Javier Mechanism of the Pseudoirreversible Binding of Amantadine to the M2 Proton Channel (Journal Article) In: Journal of the American Chemical Society, vol. 138, no. 47, pp. 15345–15358, 2016, ISSN: 1520-5126. @article{Llabr_s_2016,
title = {Mechanism of the Pseudoirreversible Binding of Amantadine to the M2 Proton Channel},
author = {Salomé Llabrés and Jordi Juárez-Jiménez and Matteo Masetti and Rosana Leiva and Santiago Vázquez and Sabrina Gazzarrini and Anna Moroni and Andrea Cavalli and F. Javier Luque},
url = {http://dx.doi.org/10.1021/jacs.6b07096},
doi = {10.1021/jacs.6b07096},
issn = {1520-5126},
year = {2016},
date = {2016-11-01},
urldate = {2016-11-01},
journal = {Journal of the American Chemical Society},
volume = {138},
number = {47},
pages = {15345–15358},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Pietro, Ornella Di; Alencar, Nelson; Esteban, Gerard; Viayna, Elisabet; Szałaj, Natalia; Vázquez, Javier; Juárez-Jiménez, Jordi; Sola, Irene; Pérez, Belén; Solé, Montse; Unzeta, Mercedes; Muñoz-Torrero, Diego; Luque, F. Javier Design, synthesis and biological evaluation of N-methyl-N-[(1,2,3-triazol-4-yl)alkyl]propargylamines as novel monoamine oxidase B inhibitors (Journal Article) In: Bioorganic & Medicinal Chemistry, vol. 24, no. 20, pp. 4835–4854, 2016, ISSN: 0968-0896. @article{Di_Pietro_2016,
title = {Design, synthesis and biological evaluation of N-methyl-N-[(1,2,3-triazol-4-yl)alkyl]propargylamines as novel monoamine oxidase B inhibitors},
author = {Ornella Di Pietro and Nelson Alencar and Gerard Esteban and Elisabet Viayna and Natalia Szałaj and Javier Vázquez and Jordi Juárez-Jiménez and Irene Sola and Belén Pérez and Montse Solé and Mercedes Unzeta and Diego Muñoz-Torrero and F. Javier Luque},
url = {http://dx.doi.org/10.1016/j.bmc.2016.06.045},
doi = {10.1016/j.bmc.2016.06.045},
issn = {0968-0896},
year = {2016},
date = {2016-10-01},
journal = {Bioorganic & Medicinal Chemistry},
volume = {24},
number = {20},
pages = {4835–4854},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Mey, Antonia S. J. S.; Juárez-Jiménez, Jordi; Hennessy, Alexis; Michel, Julien Blinded predictions of binding modes and energies of HSP90-α ligands for the 2015 D3R grand challenge (Journal Article) In: Bioorganic & Medicinal Chemistry, vol. 24, no. 20, pp. 4890–4899, 2016, ISSN: 0968-0896. @article{Mey_2016,
title = {Blinded predictions of binding modes and energies of HSP90-α ligands for the 2015 D3R grand challenge},
author = {Antonia S. J. S. Mey and Jordi Juárez-Jiménez and Alexis Hennessy and Julien Michel},
url = {http://dx.doi.org/10.1016/j.bmc.2016.07.044},
doi = {10.1016/j.bmc.2016.07.044},
issn = {0968-0896},
year = {2016},
date = {2016-10-01},
journal = {Bioorganic & Medicinal Chemistry},
volume = {24},
number = {20},
pages = {4890–4899},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Rey-Carrizo, Matias; Gazzarrini, Sabrina; Llabrés, Salomé; Frigolé-Vivas, Marta; Juárez-Jiménez, Jordi; Font-Bardia, Mercè; Naesens, Lieve; Moroni, Anna; Luque, F. Javier; Vázquez, Santiago New polycyclic dual inhibitors of the wild type and the V27A mutant M2 channel of the influenza A virus with unexpected binding mode (Journal Article) In: European Journal of Medicinal Chemistry, vol. 96, pp. 318–329, 2015, ISSN: 0223-5234. @article{Rey_Carrizo_2015,
title = {New polycyclic dual inhibitors of the wild type and the V27A mutant M2 channel of the influenza A virus with unexpected binding mode},
author = {Matias Rey-Carrizo and Sabrina Gazzarrini and Salomé Llabrés and Marta Frigolé-Vivas and Jordi Juárez-Jiménez and Mercè Font-Bardia and Lieve Naesens and Anna Moroni and F. Javier Luque and Santiago Vázquez},
url = {http://dx.doi.org/10.1016/j.ejmech.2015.04.030},
doi = {10.1016/j.ejmech.2015.04.030},
issn = {0223-5234},
year = {2015},
date = {2015-05-01},
journal = {European Journal of Medicinal Chemistry},
volume = {96},
pages = {318–329},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Rodríguez-Amigo, Beatriz; Delcanale, Pietro; Rotger, Gabriel; Juárez-Jiménez, Jordi; Abbruzzetti, Stefania; Summer, Andrea; Agut, Montserrat; Luque, F. Javier; Nonell, Santi; Viappiani, Cristiano The complex of hypericin with β-lactoglobulin has antimicrobial activity with potential applications in dairy industry (Journal Article) In: Journal of Dairy Science, vol. 98, no. 1, pp. 89–94, 2015, ISSN: 0022-0302. @article{Rodr_guez_Amigo_2015,
title = {The complex of hypericin with β-lactoglobulin has antimicrobial activity with potential applications in dairy industry},
author = {Beatriz Rodríguez-Amigo and Pietro Delcanale and Gabriel Rotger and Jordi Juárez-Jiménez and Stefania Abbruzzetti and Andrea Summer and Montserrat Agut and F. Javier Luque and Santi Nonell and Cristiano Viappiani},
url = {http://dx.doi.org/10.3168/jds.2014-8691},
doi = {10.3168/jds.2014-8691},
issn = {0022-0302},
year = {2015},
date = {2015-01-01},
journal = {Journal of Dairy Science},
volume = {98},
number = {1},
pages = {89–94},
publisher = {American Dairy Science Association},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
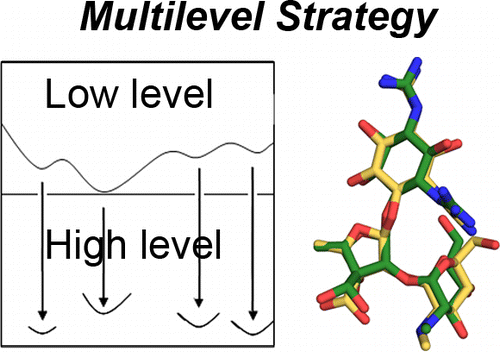
Juárez-Jiménez, Jordi; Barril, Xavier; Orozco, Modesto; Pouplana, Ramon; Luque, F. Javier Assessing the Suitability of the Multilevel Strategy for the Conformational Analysis of Small Ligands (Journal Article) In: The Journal of Physical Chemistry B, vol. 119, no. 3, pp. 1164–1172, 2014, ISSN: 1520-5207. @article{Ju_rez_Jim_nez_2014,
title = {Assessing the Suitability of the Multilevel Strategy for the Conformational Analysis of Small Ligands},
author = {Jordi Juárez-Jiménez and Xavier Barril and Modesto Orozco and Ramon Pouplana and F. Javier Luque},
url = {http://dx.doi.org/10.1021/jp506779y},
doi = {10.1021/jp506779y},
issn = {1520-5207},
year = {2014},
date = {2014-10-01},
urldate = {2014-10-01},
journal = {The Journal of Physical Chemistry B},
volume = {119},
number = {3},
pages = {1164–1172},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Pietro, Ornella Di; Pérez-Areales, F. Javier; Juárez-Jiménez, Jordi; Espargaró, Alba; Clos, M. Victòria; Pérez, Belén; Lavilla, Rodolfo; Sabaté, Raimon; Luque, F. Javier; Muñoz-Torrero, Diego Tetrahydrobenzo[h][1,6]naphthyridine-6-chlorotacrine hybrids as a new family of anti-Alzheimer agents targeting β-amyloid, tau, and cholinesterase pathologies (Journal Article) In: European Journal of Medicinal Chemistry, vol. 84, pp. 107–117, 2014, ISSN: 0223-5234. @article{Di_Pietro_2014,
title = {Tetrahydrobenzo[h][1,6]naphthyridine-6-chlorotacrine hybrids as a new family of anti-Alzheimer agents targeting β-amyloid, tau, and cholinesterase pathologies},
author = {Ornella Di Pietro and F. Javier Pérez-Areales and Jordi Juárez-Jiménez and Alba Espargaró and M. Victòria Clos and Belén Pérez and Rodolfo Lavilla and Raimon Sabaté and F. Javier Luque and Diego Muñoz-Torrero},
url = {http://dx.doi.org/10.1016/j.ejmech.2014.07.021},
doi = {10.1016/j.ejmech.2014.07.021},
issn = {0223-5234},
year = {2014},
date = {2014-09-01},
journal = {European Journal of Medicinal Chemistry},
volume = {84},
pages = {107–117},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Rey-Carrizo, Matias; Barniol-Xicota, Marta; Ma, Chunlong; Frigolé-Vivas, Marta; Torres, Eva; Naesens, Lieve; Llabrés, Salomé; Juárez-Jiménez, Jordi; Luque, Francisco J.; DeGrado, William F.; Lamb, Robert A.; Pinto, Lawrence H.; Vázquez, Santiago Easily Accessible Polycyclic Amines that Inhibit the Wild-Type and Amantadine-Resistant Mutants of the M2 Channel of Influenza A Virus (Journal Article) In: Journal of Medicinal Chemistry, vol. 57, no. 13, pp. 5738–5747, 2014, ISSN: 1520-4804. @article{Rey_Carrizo_2014,
title = {Easily Accessible Polycyclic Amines that Inhibit the Wild-Type and Amantadine-Resistant Mutants of the M2 Channel of Influenza A Virus},
author = {Matias Rey-Carrizo and Marta Barniol-Xicota and Chunlong Ma and Marta Frigolé-Vivas and Eva Torres and Lieve Naesens and Salomé Llabrés and Jordi Juárez-Jiménez and Francisco J. Luque and William F. DeGrado and Robert A. Lamb and Lawrence H. Pinto and Santiago Vázquez},
url = {http://dx.doi.org/10.1021/jm5005804},
doi = {10.1021/jm5005804},
issn = {1520-4804},
year = {2014},
date = {2014-06-01},
journal = {Journal of Medicinal Chemistry},
volume = {57},
number = {13},
pages = {5738–5747},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Viayna, Elisabet; Sola, Irene; Bartolini, Manuela; Simone, Angela De; Tapia-Rojas, Cheril; Serrano, Felipe G.; Sabaté, Raimon; Juárez-Jiménez, Jordi; Pérez, Belén; Luque, F. Javier; Andrisano, Vincenza; Clos, M. Victòria; Inestrosa, Nibaldo C.; Muñoz-Torrero, Diego Synthesis and Multitarget Biological Profiling of a Novel Family of Rhein Derivatives As Disease-Modifying Anti-Alzheimer Agents (Journal Article) In: Journal of Medicinal Chemistry, vol. 57, no. 6, pp. 2549–2567, 2014, ISSN: 1520-4804. @article{Viayna_2014,
title = {Synthesis and Multitarget Biological Profiling of a Novel Family of Rhein Derivatives As Disease-Modifying Anti-Alzheimer Agents},
author = {Elisabet Viayna and Irene Sola and Manuela Bartolini and Angela De Simone and Cheril Tapia-Rojas and Felipe G. Serrano and Raimon Sabaté and Jordi Juárez-Jiménez and Belén Pérez and F. Javier Luque and Vincenza Andrisano and M. Victòria Clos and Nibaldo C. Inestrosa and Diego Muñoz-Torrero},
url = {http://dx.doi.org/10.1021/jm401824w},
doi = {10.1021/jm401824w},
issn = {1520-4804},
year = {2014},
date = {2014-03-01},
urldate = {2014-03-01},
journal = {Journal of Medicinal Chemistry},
volume = {57},
number = {6},
pages = {2549–2567},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Juárez-Jiménez, Jordi; Mendes, Eduarda; Galdeano, Carles; Martins, Carla; Silva, Daniel B.; Marco-Contelles, José; Carreiras, Maria Carmo; Luque, F. Javier; Ramsay, Rona R. Exploring the structural basis of the selective inhibition of monoamine oxidase A by dicarbonitrile aminoheterocycles: Role of Asn181 and Ile335 validated by spectroscopic and computational studies (Journal Article) In: Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, vol. 1844, no. 2, pp. 389–397, 2014, ISSN: 1570-9639. @article{Ju_rez_Jim_nez_2014b,
title = {Exploring the structural basis of the selective inhibition of monoamine oxidase A by dicarbonitrile aminoheterocycles: Role of Asn181 and Ile335 validated by spectroscopic and computational studies},
author = {Jordi Juárez-Jiménez and Eduarda Mendes and Carles Galdeano and Carla Martins and Daniel B. Silva and José Marco-Contelles and Maria Carmo Carreiras and F. Javier Luque and Rona R. Ramsay},
url = {http://dx.doi.org/10.1016/j.bbapap.2013.11.003},
doi = {10.1016/j.bbapap.2013.11.003},
issn = {1570-9639},
year = {2014},
date = {2014-02-01},
journal = {Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics},
volume = {1844},
number = {2},
pages = {389–397},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Pietro, Ornella Di; Viayna, Elisabet; Vicente-García, Esther; Bartolini, Manuela; Ramón, Rosario; Juárez-Jiménez, Jordi; Clos, M. Victòria; Pérez, Belén; Andrisano, Vincenza; Luque, F. Javier; Lavilla, Rodolfo; Muñoz-Torrero, Diego 1,2,3,4-Tetrahydrobenzo[h][1,6]naphthyridines as a new family of potent peripheral-to-midgorge-site inhibitors of acetylcholinesterase: Synthesis, pharmacological evaluation and mechanistic studies (Journal Article) In: European Journal of Medicinal Chemistry, vol. 73, pp. 141–152, 2014, ISSN: 0223-5234. @article{Di_Pietro_2014b,
title = {1,2,3,4-Tetrahydrobenzo[h][1,6]naphthyridines as a new family of potent peripheral-to-midgorge-site inhibitors of acetylcholinesterase: Synthesis, pharmacological evaluation and mechanistic studies},
author = {Ornella Di Pietro and Elisabet Viayna and Esther Vicente-García and Manuela Bartolini and Rosario Ramón and Jordi Juárez-Jiménez and M. Victòria Clos and Belén Pérez and Vincenza Andrisano and F. Javier Luque and Rodolfo Lavilla and Diego Muñoz-Torrero},
url = {http://dx.doi.org/10.1016/j.ejmech.2013.12.008},
doi = {10.1016/j.ejmech.2013.12.008},
issn = {0223-5234},
year = {2014},
date = {2014-02-01},
journal = {European Journal of Medicinal Chemistry},
volume = {73},
pages = {141–152},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Bolea, Irene; Juárez-Jiménez, Jordi; Rı́os, Cristóbal; Chioua, Mourad; Pouplana, Ramón; Luque, F. Javier; Unzeta, Mercedes; Marco-Contelles, José; Samadi, Abdelouahid Synthesis, Biological Evaluation, and Molecular Modeling of Donepezil and N-[(5-(Benzyloxy)-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine Hybrids as New Multipotent Cholinesterase/Monoamine Oxidase Inhibitors for the Treatment of Alzheimer’s Disease (Journal Article) In: Journal of Medicinal Chemistry, vol. 54, no. 24, pp. 8251–8270, 2011, ISSN: 1520-4804. @article{Bolea_2011,
title = {Synthesis, Biological Evaluation, and Molecular Modeling of Donepezil and N-[(5-(Benzyloxy)-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine Hybrids as New Multipotent Cholinesterase/Monoamine Oxidase Inhibitors for the Treatment of Alzheimer’s Disease},
author = {Irene Bolea and Jordi Juárez-Jiménez and Cristóbal Rı́os and Mourad Chioua and Ramón Pouplana and F. Javier Luque and Mercedes Unzeta and José Marco-Contelles and Abdelouahid Samadi},
url = {http://dx.doi.org/10.1021/jm200853t},
doi = {10.1021/jm200853t},
issn = {1520-4804},
year = {2011},
date = {2011-11-01},
urldate = {2011-11-01},
journal = {Journal of Medicinal Chemistry},
volume = {54},
number = {24},
pages = {8251–8270},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|
Duque, María D.; Ma, Chunlong; Torres, Eva; Wang, Jun; Naesens, Lieve; Juárez-Jiménez, Jordi; Camps, Pelayo; Luque, F. Javier; DeGrado, William F.; Lamb, Robert A.; Pinto, Lawrence H.; Vázquez, Santiago Exploring the Size Limit of Templates for Inhibitors of the M2 Ion Channel of Influenza A Virus (Journal Article) In: Journal of Medicinal Chemistry, vol. 54, no. 8, pp. 2646–2657, 2011, ISSN: 1520-4804. @article{Duque_2011,
title = {Exploring the Size Limit of Templates for Inhibitors of the M2 Ion Channel of Influenza A Virus},
author = {María D. Duque and Chunlong Ma and Eva Torres and Jun Wang and Lieve Naesens and Jordi Juárez-Jiménez and Pelayo Camps and F. Javier Luque and William F. DeGrado and Robert A. Lamb and Lawrence H. Pinto and Santiago Vázquez},
url = {http://dx.doi.org/10.1021/jm101334y},
doi = {10.1021/jm101334y},
issn = {1520-4804},
year = {2011},
date = {2011-04-01},
journal = {Journal of Medicinal Chemistry},
volume = {54},
number = {8},
pages = {2646–2657},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
|

![Synthesis, Biological Evaluation, and Molecular Modeling of Donepezil and N-[(5-(Benzyloxy)-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine Hybrids as New Multipotent Cholinesterase/Monoamine Oxidase Inhibitors for the Treatment of Alzheimer’s Disease](https://www.ub.edu/cmd_lab/wp-content/uploads/2024/02/MAOB-1.png)
