Ghashghaei, O.; Caputo, S.; Sintes, M.; Revés, M.; Kielland, N.; Estarellas, C.; Luque, F.J.; Aviñó, A.; Eritja, R.; Serna-Gallego, A.; Marrugal-Lorenzo, J. A.; Pachón, J.; Sánchez-Céspedes, J.; Treadwell, R.; de Moliner, F.; Vendrell, M.; Lavilla, R. Chemistry, a European Journal, 2018, 24, 14513-14521
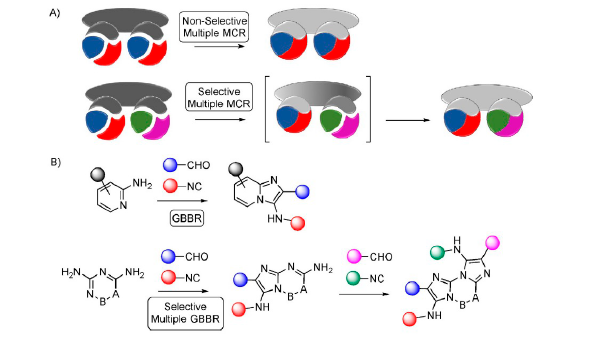
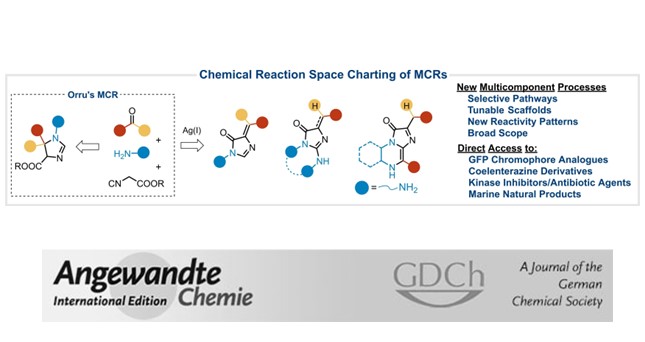
Multiple multicomponent reactions rapidly assemble complex structures. Despite being very productive, the lack of selectivity and the reduced number of viable transformations restrict their general application in synthesis. Hereby, we describe a rationale for a selective version of these processes based in the preferential generation of intermediates which are less reactive than the initial substrates. In this way, applying the Groebke–Blackburn–Bienaymé reaction on a range of α-polyamino-polyazines, we prepared a family compact heterocyclic scaffolds with relevant applications in medicinal and biological chemistry (live cell imaging probes, selective binders for DNA quadruplexes, and antiviral agents against human adenoviruses). The approach has general character and yields complex molecular targets in a selective, tunable and direct manner.
https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/chem.201802877