Mona Peyman, Emma Barroso, Andreea L. Turcu, Francesc Estrany Jr., Dáire Smith, Javier Jurado-Aguilar, Patricia Rada, Christophe Morisseau, Bruce D. Hammock, Ángela M. Valverde, Xavier Palomer, Carles Galdeano, Santiago Vázquez, Manuel Vázquez-Carrera
DOI: 10.1016/j.biopha.2023.115667
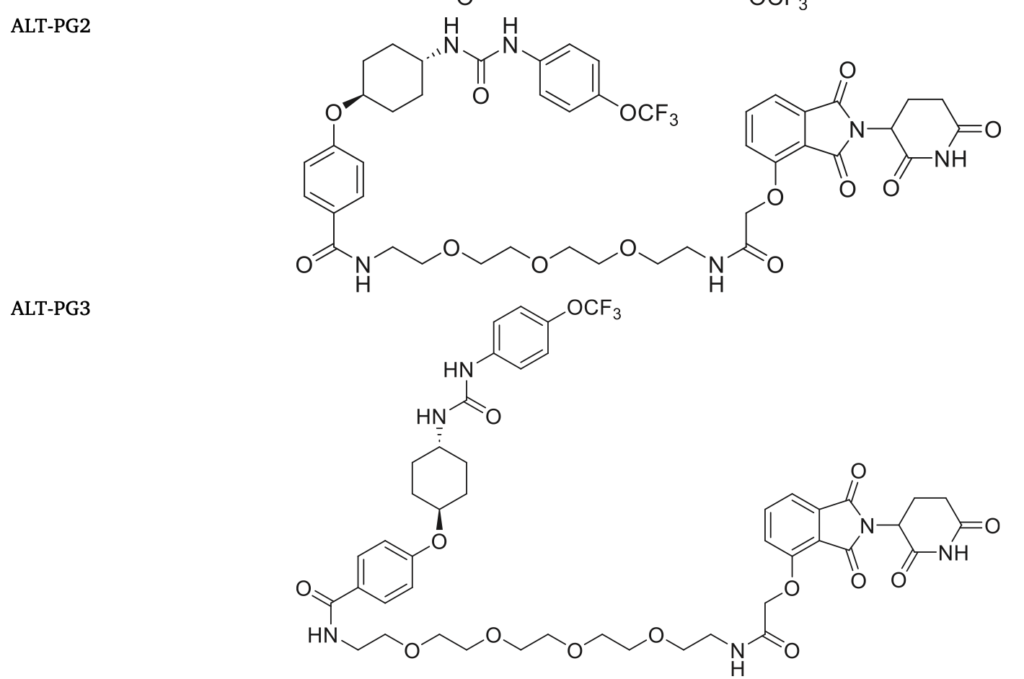
Soluble epoxide hydrolase (sEH) is a drug target with the potential for therapeutic utility in the areas of inflammation, neurodegenerative disease, chronic pain, and diabetes, among others. Proteolysis-targeting chimeras (PROTACs) mols. offer new opportunities for targeting sEH, due to its capacity to induce its degradation Here, we describe that the new ALT-PG2, a PROTAC that degrades sEH protein in the human hepatic Huh-7 cell line, in isolated mouse primary hepatocytes, and in the liver of mice. Remarkably, sEH degradation caused by ALT-PG2 was accompanied by an increase in the phosphorylated levels of AMP-activated protein kinase (AMPK), while phosphorylated extracellular-signal-regulated kinase 1/2 (ERK1/2) was reduced. Consistent with the key role of these kinases on endoplasmic reticulum (ER) stress, ALT-PG2 attenuated the levels of ER stress and inflammatory markers. Overall, the findings of this study indicate that targeting sEH with degraders is a promising pharmacol. strategy to promote AMPK activation and to reduce ER stress and inflammation.