Stampolaki, Marianna; Malwal, Satish R.; Alvarez-Cabrera, Nadine; Gao, Zijun; Moniruzzaman, Mohammad; Babii, Svitlana O.; Naziris, Nikolaos; Rey-Cibati, Andre; Valladares-Delgado, Mariana; Turcu, Andreea L.; Baek, Kyung-Hwa; Phan, Trong-Nhat; Lee, Hyeryon; Alcaraz, Mattheo; Watson, Savannah; van der Watt, Mariette; Coertzen, Dina; Efstathiou, Natasa; Stylianakis, Ioannis; Chountoulesi, Maria; Shoen, Carolyn M.; Papanastasiou, Ioannis P.; Brea, Jose; Cynamon, Michael H.; Birkholtz, Lyn-Marie; Kremer, Laurent; No, Joo Hwan; Vazquez, Santiago; Benaim, Gustavo; Demetzos, Costas; Zgurskaya, Helen I.; Dick, Thomas; Oldfield, Eric; Kolocouris, Antonios D.
DOI: 10.26434/chemrxiv-2022-xm94p
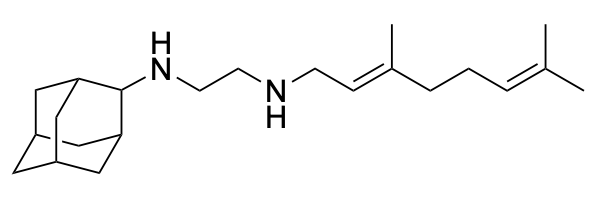
SQ109 is a tuberculosis drug candidate that has high potency against Mycobacterium tuberculosis and is thought to function at least in part by blocking cell wall biosynthesis by inhibiting the MmpL3 transporter. It also has activity against bacteria and protozoan parasites that lack MmpL3, where it can act as an uncoupler, targeting lipid membranes and Ca2+ homeostasis. Here, we synthesized 19 analogs of SQ109 and tested them against bacteria: M. smegmatis, M. tuberculosis, M. abscessus, Bacillus subtilis and Escherichia coli, as well as against the protozoan parasites, Trypanosoma brucei, T. cruzi, Leishmania donovani, L. mexicana and Plasmodium falciparum. Activity against the mycobacteria was generally less than with SQ109 and was reduced by increasing the size of the alkyl adduct, but two analogs were ~4-8 fold more active than was SQ109 against M. abscessus, including a highly drug resistant strain harboring a A309P mutation in MmpL3. There was also better activity than found with SQ109 with other bacteria and protozoa. Of particular interest, we found that the adamantyl C-2 ethyl, butyl, phenyl and benzyl analogs had 4-10x increased activity against P. falciparum asexual blood stages, together with low toxicity to a human HepG2 cell line, making them of interest as new anti-malarial drug leads. We also used surface plasmon resonance to investigate the binding of inhibitors to MmpL3, and differential scanning calorimetry to investigate binding to lipid membranes. There was no correlation between MmpL3 binding and M. tuberculosis or M. smegmatis cell activity, suggesting that MmpL3 is not a major target, in mycobacteria. However, some of the more active species decreased lipid phase transition temperatures, indicating increased accumulation in membranes, expected to lead to enhanced uncoupler activity.