Rodríguez-Arévalo S, Pujol E, Abás S, Galdeano C, Escolano C, Vázquez S.
Molecules. 2021, 26, 906. doi: 10.3390/molecules26040906.
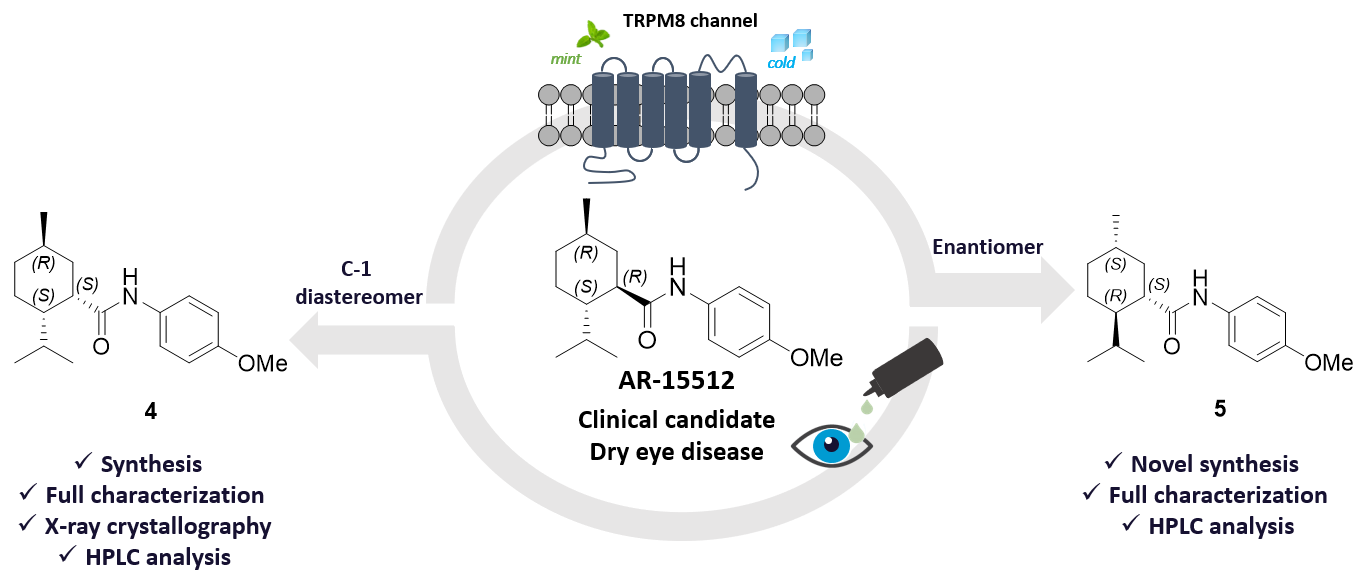
AR-15512 (formerly known as AVX-012 and WS-12) is a TRPM8 receptor agonist currently in phase 2b clinical trials for the treatment of dry eye. This bioactive compound with menthol-like cooling activity has three stereogenic centers, and its final structure and absolute configuration, (1R,2S,5R), have been previously solved by cryo-electron microscopy. The route of synthesis of AR-15512 has also been reported, revealing that epimerization processes at the C-1 can occur at specific stages of the synthesis. In order to confirm that the desired configuration of AR-15512 does not change throughout the process and to discard the presence of the enantiomer in the final product due to possible contamination of the initial starting material, both the enantiomer of AR-15512 and the diastereomer at the C-1 were synthesized and fully characterized. In addition, the absolute configuration of the (1S,2S,5R)-diastereomer was determined by X-ray crystallographic analysis, and new HPLC methods were designed and developed for the identification of the two stereoisomers and their comparison with the clinical candidate AR-15512.