Healthy ageing is becoming increasingly threatened by a number of age-related neurodegenerative diseases, including Alzheimer’s disease (AD). AD causes loss of memory and other intellectual abilities, seriously interfering with daily life quality, and finally it inexorably causes the death of the patient. Over 50 million people are currently suffering AD and, unlike other major diseases whose mortality has significantly decreased in the past decade (i.e., breast or prostate cancer, heart disease, stroke or HIV), the number of AD-related deaths increased by 145% in the past two decades. Likewise, the economic burden associated to AD shows a clear rising trend, currently accounting for > 1% of the global gross domestic product. These figures will rise in the upcoming years along with the increase in life expectancy, thereby posing a huge pressure on society and health systems.
The elusiveness of efficacious drugs against AD greatly accounts for its fatal consequences. So far, only four drugs have been globally approved for the palliative treatment of AD. Two monoclonal antibodies directed to the β-amyloid (Aβ) peptide, thought to be the main culprit of the AD, have been recently approved by the FDA but one of them was rejected by EMA due to lack of efficacy issues and the other one is still pending approval. Indeed, a large number of drug candidates directed to Aβ have failed to show efficacy in advanced clinical trials. This has led to the credence that Aβ is just one of the causes of AD, which might involve a complex pathological network where several key targets are jointly responsible for the neurodegeneration. Accordingly, AD could be efficiently treated only through the simultaneous modulation of several key targets.
In our research group we are synthesizing novel anti-Alzheimer drug candidates, which have been rationally designed to hit multiple key biological targets involved in the pathological network of AD such as BACE-1, Aβ and tau aggregation, MAO-B, oxidative stress, soluble epoxide hydrolase, and cholinesterases, among others.



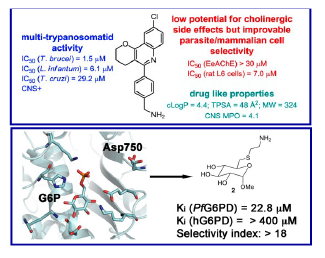
Figure 1. A walk along the active site gorge of acetylcholinesterase: from extended binding active site inhibitors to multisite inhibitors and multitarget agents (image from Acc. Chem. Res. 2024, 57, 450-467), target combinations pursued in the multitarget anti-Alzheimer compounds developed in the period 1990-2020 (image from Pharmaceuticals 2022, 15, 545) and chemical structures and in vitro and in vivo biological profile of four multitarget anti-Alzheimer leads developed in our research group (images from J. Med. Chem. 2022, 65, 4909-4925, Eur. J. Med. Chem. 2021, 225, 113779, J. Med. Chem. 2021, 64, 812-839, and J. Med. Chem. 2020, 63, 9360-9390).